The DiGA-Fast-Track | New opportunities for healthcare innovators in Germany
Getting your digital health app ready for reimbursement in Germany
With the Digital Health Care Act (Digitale-Versorgung-Gesetz, DVG), Digital Health Apps (Digitale Gesundheitsanwendungen, DiGA) haven been added to the benefits and services covered by Germany’s statutory health insurance (SHI). Just like prescription drugs, DiGA can be prescribed by doctors and psychotherapists to their patients. Thanks to these new “prescription apps”, digital health companies now have the chance to access a reimbursement market of 73 million publicly insured potential health app users.
But to navigate from the first product idea to successful market entry, companies need to meet regulatory requirements and to watch out for potential pitfalls.
Click to find out more about DiGA market access:
- Digital Health Care Act and DiGA Fast-Track
- DiGA criteria
- Regulatory DiGA requirements
(DiGA guide and DiGA ordinance, DiGAV) - Study design and evaluation strategy
- Proof of positive healthcare effects
- DiGA pricing
- Health app reimbursement options beyond DiGA
- Modular digital health consulting at _fbeta
Consulting and Support
DiGA Fast Track
Digital Health Care Act and DiGA Fast Track
Digital Health Care Act and DiGA Fast Track
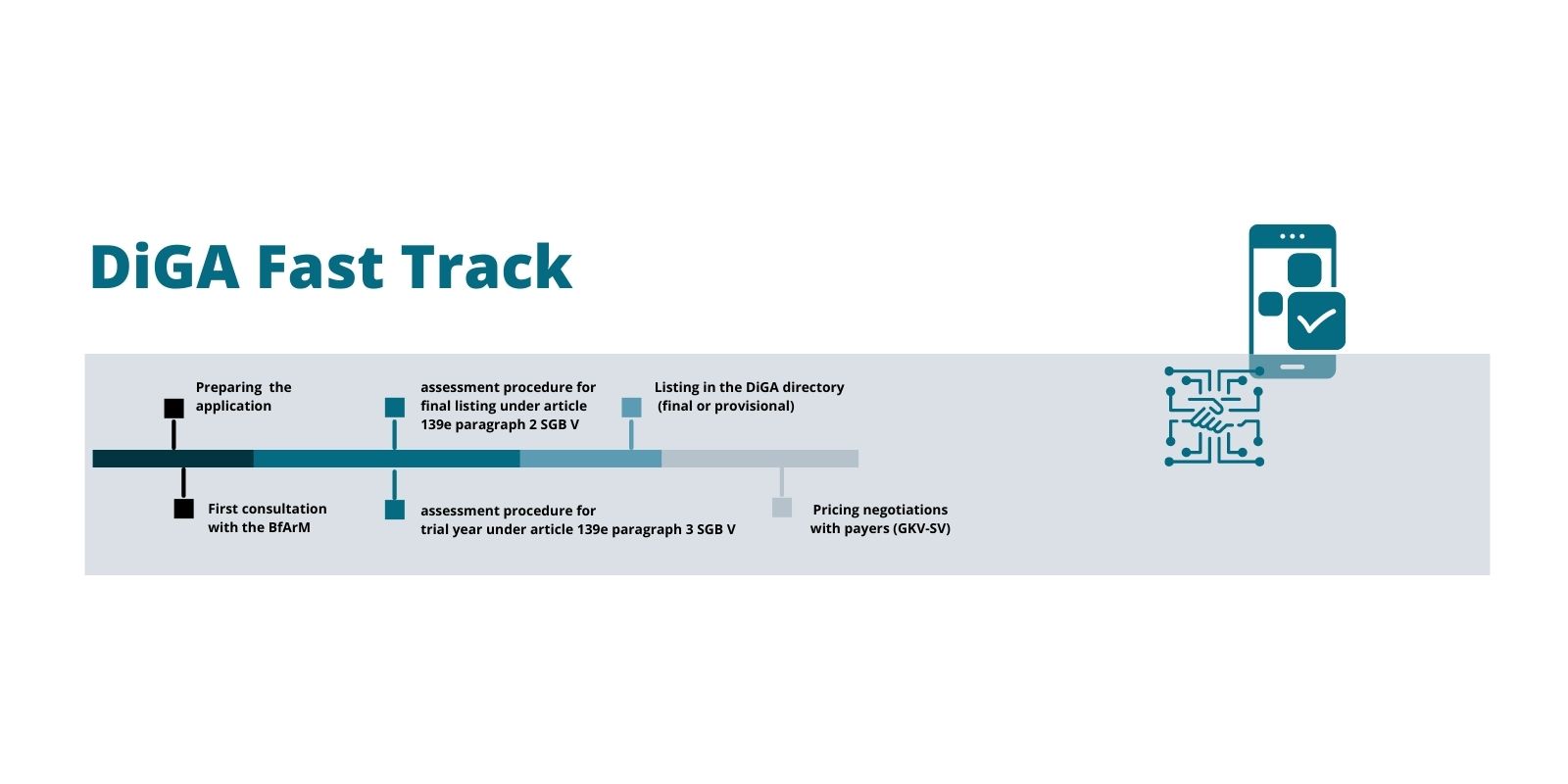
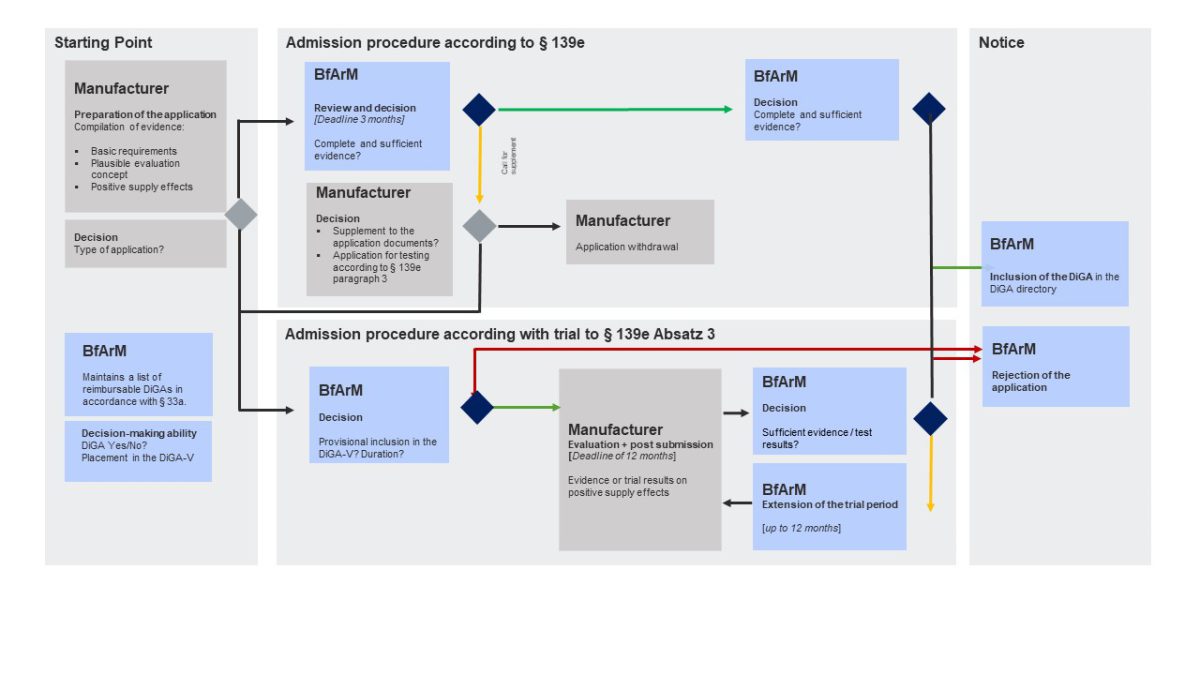
The German Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM) is in charge of DiGA assessment and approval. The BfArM’s assessment procedure opens up a new way for digital health apps to enter the reimbursement market. A DiGA that has successfully completed the assessment procedure is listed in a register of prescription health apps, the DiGA directory.
The DiGA assessment follows a Fast Track to market entry and reimbursement: The BfArM decides on the approval of a digital health app within three months of receiving the manufacturer’s application. Digital health companies can either apply for final or for provisional listing (under article 139e paragraphs 2 and 3 Book V of the Social Code, SGB V).
A provisional listing kicks off a 1-year trial phase after which the manufacturer needs to provide proof of “positive healthcare effects” (positive Versorgungseffekte, pVE) as a basis for the final listing. The DiGA can be prescribed and is reimbursed during the entire trial year. Thus, the DiGA fast track with its possibility of a trial year is a great opportunity for accelerated market access and to offset some of the development costs already during the clinical trial phase. Time to market can be accelerated even more with purposeful planning and preparation.
Criteria Defining a DiGA
Criteria Defining a DiGA
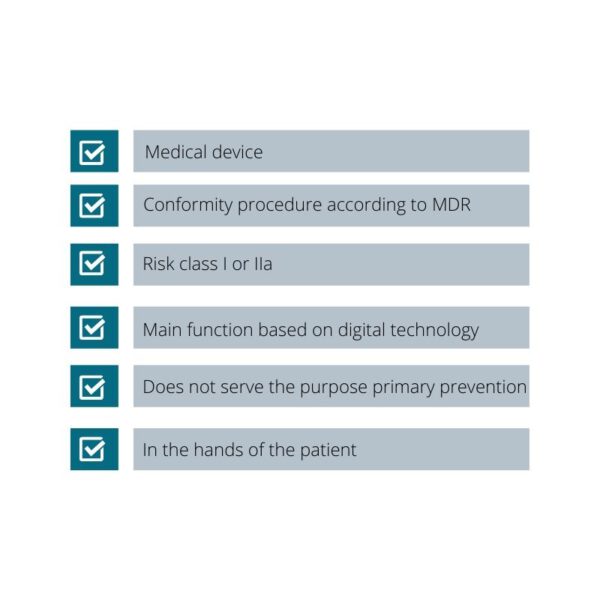
DiGA are a class I or class IIa medical device by European MDR (Medical Device Regulation) classification rules. DiGA support the detection, monitoring, treatment, or alleviation of diseases or the detection, treatment, alleviation, or compensation of injuries or disabilities. A DiGA’s main function is based on its digital technology. A DiGA does not not only serve the purpose of reading out or controlling another (medical) device; a DiGA’s medical intended use is achieved via its digital main function. A DiGA does not serve the purpose of primary prevention. DiGA should mainly be used by the patient, but can also be used by both the patient and healthcare providers together. These criteria are defined in § 33a SGB V.
Regulatory DiGA Requirements
Regulatory DiGA Requirements
To be listed in the DiGA directory under § 139e SGB V, each DiGA needs to conform to the requirements of §§ 3 to 6 of the DiGA ordinance (DiGA-Verordnung, DiGAV).
DiGA need to comply with regulatory requirements pertaining to:
- Safety and suitability for use,
- data protection and information security,
- quality, and in particular interoperability.
The CE marking under the MDR regulation confirms that the digital health app complies with requirements regarding safety and suitability for use. The checklists found in DiGAV annexes 1 and 2 can be used to self-declare compliance with the remaining requirements.
Insights
How to apply the DiGA requirements to a specific product or DiGA concept is not entirely set in stone. Make use of the freedom that both the DiGA ordinance and the DiGA guide leave you. Being well-prepared for the pivotal first consultation with the BfArM maximizes your chances of a successful and speedy approval.
Study Design and Evaluation Strategy
Study Design and Evaluation Strategy
An evaluation concept is the prerequisite of a provisional listing followed by a 1-year trial phase. It has to meet generally accepted scientific standards. The evaluation concept needs to explicate for which type of “positive healthcare effect” (pVE) evidence is to be provided, for which patient population, and why the DiGA is suited achieve the effect. In particular, the evaluation concept should establish the planned study design, the comparator with regard to the day-to-day reality of healthcare, and the chosen trial outcomes. It also needs to specify how evidence is to be produced within the 1-year trial phase.
The evaluation concept must include a study protocol and a statistical analysis plan. It also needs to adequately include and take into account the results of systematic data analysis. Data to be analyzed consist both in a systematic literature research and in data collected with the app itself. The data analysis must demonstrate to the BfArM that the app can achieve the intended positive healthcare effect for the chosen patient group. The analyzed data also provide useful information to define study parameters for the trial period, such as effect size, case number, outcome measurement instruments, and recruitment methods.
The minimum requirement for a DiGA study is a retrospective comparative study. For instance, case-control-studies, retrospective cohort studies, and intraindividual comparisons are all acceptable study types for DiGA. The DiGA manufacturer itself is free to choose a study type of a higher evidence level (see chapter 6 of the DiGA guide). However, the BfArM as well may request a prospective study if the validity of retrospective data is questionable. The DiGA study is to be conducted in Germany. If not, the manufacturer must provide proof that that the healthcare context in which the study was conducted is comparable to the one in Germany. DiGA studies should reflect the reality of German healthcare. The obtained results must be quantitative.
The study is to be registered in a public trial register (e.g., the DRKS register). Complete study results are to be published no later than 12 months after study completion.

Insights
Just like any software, software as a medical device tends to be characterized by agile development, frequent new releases, and tight budgets. Not just for the development of the DiGA software, but also for the evaluation concept, it is key to look ahead and to adapt the original plan if need be. Our recommendations:
- Analyzing and reporting trial data for your application take time. Accommodate for that in your project plan!
- The evaluation concept is an important document to focus on as it is needed for the application. But you cannot focus on the evaluation concept alone. Don’t lose sight of all the other relevant aspects and processes pertaining to market access. Plan ahead!
- When starting to collect data with the app, the app-development should be almost completed. We know from experience that the BfArM requires that the app data are collected with a product that is comparable to the to-be-listed DiGA. Thus, before starting to collect data with your app, make sure that you won’t need to make significant changes later!
Find more insights and learnings in our article „Evaluationskonzept reloaded – Think ahead!“ (in German)
Proof of Positive Healthcare Effects
Proof of Positive Healthcare Effects
With a (final or provisional) listing in the DiGA directory, digital health apps are reimbursable within Germany’s SHI system. One key element of the DiGA Fast-Track assessment procedure focuses on the DiGA’s positive healthcare effect.
The term of positive healthcare effects (pVE) comprises both medical benefits (medizinischer Nutzen, mN) and as patient-relevant improvement of structures and processes (patientenrelevante Struktur- und Verfahrensverbesserungen, pSVV). Before the passing of the Digital Health Care Act, pSVV played no role with regard to the reimbursement of a service or benefit in Germany. With the introduction of pSVV into the German SHI system, lawmakers have endorsed the idea that DiGA can improve healthcare structures and processes and that this type of benefit should justify reimbursement – also in the absence of a medical benefit.
Manufacturers who have already proven their DiGA’s positive healthcare effects in a study can directly apply for a final listing. As an alternative, an application for a provisional listing is possible. The provisionally listed DiGA has 12 months (which can be extended by another year in justified exceptional cases) to prove its positive healthcare effects.
DiGA Pricing
DiGA Pricing
A year after a digital health app has been listed in the DiGA directory, the manufacturer’s price is superseded by a reimbursement agreement between the manufacturer and the National Association of SHI Funds (GKV-Spitzenverband, GKV-SV). If both parties cannot come to an agreement, an arbitration board will set a DiGA price under § 134 paragraph 3 SGB V. The new reimbursement price retroactively takes effect from the thirteenth month after the DiGA listing.
Some principles of DiGA pricing are defined in the master agreement pursuant to § 134 paragraphs 4 and 5 SGB V. For instance, the actual self-pay app price in Germany and from other European countries is factored into the pricing process. In addition, pricing strategies may take into account the number of delivered DiGA activation codes and pay-for-performance principles.
However, the type and amount of proven additional benefit the DiGA can provide for patients remains the most important criterion for DiGA pricing.
Health app reimbursement options beyond DiGA
Health app reimbursement options beyond DiGA
In the German healthcare system, “prescription apps” are not the only reimbursement option for digital health apps. It is often unclear to digital health companies which type of contract is most advantageous from a market access and business perspective.
„Besondere Versorgung“ (§ 140 SGB V) and other product-specific compensation models negotiated with individual payers are commonly used for market access in digital health. But other options to leverage the potential of digital health apps in the outpatient and inpatient sectors as well as in primary prevention should be considered too.
Each market access and reimbursement strategy comes with different stakeholders that need to be approached and convinced, different processes that need to be followed, and different regulatory requirements that need to be met. The time and effort for the application and approval process may also vary considerably from one type of contract to the other.
Therefore, it is important to know your reimbursement alternatives and identify the most appropriate and most advantageous market access option(s) for your product early on, so that you can consider the related processes and requirements right from the start.
We help you find out which compensation and reimbursement models best fit your idea, determine the corresponding requirements, and pave your way through to market entry.
Modular
digital health and DiGA consulting at _fbeta
It is our goal to guide, support, and empower digital health or DiGA companies in a customized yet cost-efficient manner. Our portfolio of digital health consulting modules is tailored to fit the requirements and challenges of the DiGA Fast Track.
We know the ins and outs of DiGA regulation. We have accompanied the German Federal Ministry of Health (BMG) throughout the digital health legislative process. And we have acted as a sparring partner for the Federal Institute for Drugs and Medical Devices (BfArM) regarding the application submission portal.
We also know how to navigate the process in practice, having supported and consulted more than 50 digital health companies throughout the DiGA in different stages of the application procedure and the assessment process. Thereby, we know the success factors and can anticipate the hurdles and pitfalls to make sure your journey is both safe and successful.
Our modular digital health consulting portfolio can be customized to your needs. We can offer you custom-tailored support in all aspects pertaining to reimbursable digital health apps – not only DiGAs:
Full-service support throughout the DiGA Fast Track | Fulfilment of DiGA criteria
CE marking under the Medical Device Regulation (MDR)
Proof of positive healthcare effects | Evaluation concept and study design
DiGA pricing and payer negotiations
Interoperability
Data protection and data privacy
Market entry | Marketing | Sales and Distribution | Stakeholder management
Market access beyond the DiGA Fast Track | Reimbursement and compensations options for digital health in the German SHI system
beta_ask
Book a free consultation call!
You have a specific question? You want to find out how we can help you? Be sure to book a free consultation call!
_customer





















_hub
Pointed statements, event reports and concrete how-tos – as magazine articles and videos – can be found here.