The DiGA-Fast-Track | New opportunities for healthcare innovators in Germany
The DiGA-Fast-Track | New opportunities for healthcare innovators in Germany
Getting your digital health app ready for reimbursement in Germany
With the Digital Health Care Act (Digitale-Versorgung-Gesetz, DVG), Digital Health Apps (Digitale Gesundheitsanwendungen, DiGA) haven been added to the benefits and services covered by Germany’s statutory health insurance (SHI). Just like prescription drugs, DiGA can be prescribed by doctors and psychotherapists to their patients. Thanks to these new “prescription apps”, digital health companies now have the chance to access a reimbursement market of 73 million publicly insured potential health app users.
But to navigate from the first product idea to successful market entry, companies need to meet regulatory requirements and to watch out for potential pitfalls.
We consult and support
Modular digital health consulting at _fbeta
Further information on market access
Watch now: Interview with Jonas Albert
How DiGA & DiPA work and what’s next for digital health in Germany
beta_shorts
Germany is the only country in the world with a formal Fast Track for digital health apps – giving them access to reimbursement within 12 months. In this short videos #beta_shorts , we explain what makes the DiGA Fast Track so special – and what to look out for if you’re considering it.
Modular
digital health and DiGA consulting at _fbeta
It is our goal to guide, support, and empower digital health or DiGA companies in a customized yet cost-efficient manner. Our portfolio of digital health consulting modules is tailored to fit the requirements and challenges of the DiGA Fast Track: The _fbeta all-in-one package for digital health applications.
We know the ins and outs of DiGA regulation. We have accompanied the German Federal Ministry of Health (BMG) throughout the digital health legislative process. And we have acted as a sparring partner for the Federal Institute for Drugs and Medical Devices (BfArM) regarding the application submission portal.
We also know how to navigate the process in practice, having supported and consulted more than 50 digital health companies throughout the DiGA in different stages of the application procedure and the assessment process. Thereby, we know the success factors and can anticipate the hurdles and pitfalls to make sure your journey is both safe and successful.
Our modular digital health consulting portfolio can be customized to your needs. We can offer you custom-tailored support in all aspects pertaining to reimbursable digital health apps – not only DiGAs:
Full-service support throughout the DiGA Fast Track | Fulfilment of DiGA criteria
Despite the clear definition of what is and what is not a DiGA, there are always discussions with the BfArM as to whether a product fulfills all DiGA criteria. The focus is often on the main digital function and the extent to which medical and non-physician service providers are involved.
We support you in determining whether your product is DiGA-compliant and help you to find the right design for your product so that all DiGA criteria are met. In addition, we provide you with expert support throughout the entire Fast Track application process. In addition to advice on a wide range of issues relating to the application process, this also includes the preparation and review of application documents as well as the preparation and support of consultations with the BfArM.
We are happy to support and advise you on
- Questions about the DiGA application procedure
- DiGA definition
- Fulfillment of DiGA criteria
- Product tailoring
- Preparation of consultations with the BfArM
- Technical support for DiGA application and review
CE marking under the Medical Device Regulation (MDR)
The certification of your product as a medical device in accordance with MDR is an important step on the DiGA fast track.
We accompany you through the certification process. In addition to supporting you in establishing a quality management system, we work with you to effectively implement the certification process by jointly creating the technical documentation and providing advice on implementing the standards.
Proof of positive healthcare effects | Evaluation concept and study design
Via the DVG Fast Track, DiGAs can be included in the register definitively or on a trial basis and thus become eligible for reimbursement within the SHI system. One of the central requirements of the fast track is proof of the positive effects on care. The basis for inclusion on a trial basis is the evaluation concept drawn up in accordance with generally recognized scientific standards. This must set out the positive care effects (pVE) for which evidence is to be provided for a specific patient population and how these effects are to be demonstrated during the trial period.
In particular, the evaluation concept should justify the choice of study design, the chosen comparison in the context of the reality of care and the outcomes.
We are happy to support and advise you on
- Selection of the study design
- Preparation of the study protocol incl. statistical analysis plan
- Systematic evaluation of your own DiGA data / preliminary study
- Systematic literature research and evaluation
- Preparation of the evaluation concept
DiGA pricing and payer negotiations
An appropriate price for the DiGA is elementary for the profitability and thus the sustainable success of a manufacturer. After successful inclusion in the DiGA directory, the price for the DiGA freely determined by the manufacturer initially applies for one year. After the first twelve months, the actual price is replaced by the reimbursement amount, which must be negotiated between the manufacturer and the National Association of Statutory Health Insurance Funds.
We support you in developing a suitable pricing strategy and in setting a market-driven price and prepare you for the negotiations with the National Association of Statutory Health Insurance Funds.
Interoperability
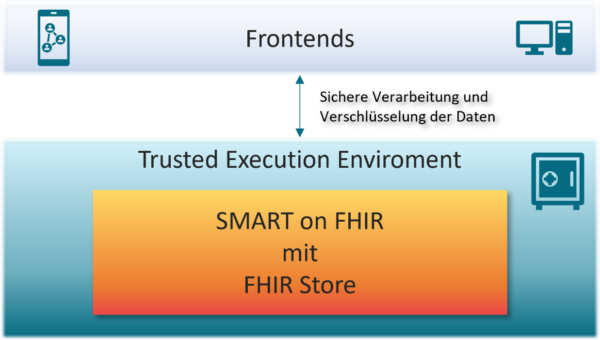
Use our tried-and-tested solutions for seamless integration of your DiGA into the telematics infrastructure (TI). Through our partnership with RISE (TI-as-a-Service provider), we provide you with quick and easy access to the TI. Building on this, we offer you all the solution modules you need to successfully implement the interoperability requirements of the DiGA regulation:
- DiGA adapter for the GesundheitsID: The GesundheitsID enables secure 2-factor authentication for DiGA users and has been mandatory for all statutory health insurance companies since 2024. The adapter we developed enables access to the GesundheitsID via a simple interface based on the OpenID Connect standard. The solution is already in use at several DiGAs and meets all the technical requirements of gematik and BSI TR-03161.
- ePA client: We support the secure connection to the electronic patient record (ePA for all) so that your DiGA can interact seamlessly with the ePA. Our solution encapsulates all ePA-internal interfaces – including VAU channel, IDP authentication and TI integration – and offers you a simple, user-friendly connection of your DiGA to the ePA for all.
- DiGA MIOs: We provide you with technical support in implementing the MIO-based export format required for inclusion in the DiGA directory. Our solution ensures full interoperability with the TI systems and makes it easier for you to comply with all regulatory requirements.
With our complete solution comprising TI connection, health ID integration and ePA connection, we offer you a flexible, modular and cost-effective solution that minimizes the technical effort and accelerates the market entry of your DiGA.
Data protection and data privacy
On the topics of data protection and data security, the DiGAV (Annex 1) specifies the requirements of the General Data Protection Regulation and other DiGA-specific data protection regulations for manufacturers.
We support and assist you in implementing the requirements in a targeted manner.
Our experts will provide you with comprehensive advice on the requirements of Annex 1 DiGAV, Annex 2 DiGAV, the BSI TR-03161 and the new data protection criteria of the BfArM. We offer both workshops and individual consulting packages for your specific questions.
- Basic auditing
- Risk minimization
- Data protection training/sensitization
- Data protection impact assessment
Market entry | Marketing | Sales and Distribution | Stakeholder management
Achieving reimbursement eligibility through inclusion in the DiGA directory is only a milestone for a DiGA. Really gaining a foothold in healthcare and among patients and establishing itself on the market are further key steps for successful market access. DiGAs are a new service area in healthcare. As a result, DiGA providers must do the groundwork: a DiGA must become visible to sales partners and users and gain their acceptance.
A DiGA-specific challenge for sales and marketing is to characterize and individually evaluate the three possible sales channels via doctors, patients or health insurance companies. Marketing measures can thus be planned and prioritized in a targeted manner. In this way, DiGA can be effectively positioned in the market with a wide reach.
We advise and support you
- Definition of the relevant target groups
- Strategy development
- Consideration of the necessary regulatory and legal framework conditions (e.g. Therapeutic Products Advertising Act)
Market access beyond the DiGA Fast Track | Reimbursement and compensations options for digital health in the German SHI system
In the German healthcare system, the DiGA fast track is not the only reimbursement option for digital health applications. Alternative reimbursement models such as selective contracts can also be a suitable option, although they are subject to different processes and requirements.
Therefore, knowledge of the entire national market environment and other regulatory frameworks surrounding the various reimbursement channels is key to successfully accessing the primary healthcare market and effectively positioning your product.
We help you to find out which reimbursement model best suits your idea, identify the relevant regulatory requirements and support you on your path to market entry.
With our modular digital health portfolio, we offer customized support in all matters relating to reimbursable digital health applications – not just DiGAs. Do you have a specific question? Would you like to find out how we can help you? Make sure you get a free consultation.
Digital Health Care Act and DiGA Fast Track
Further Information
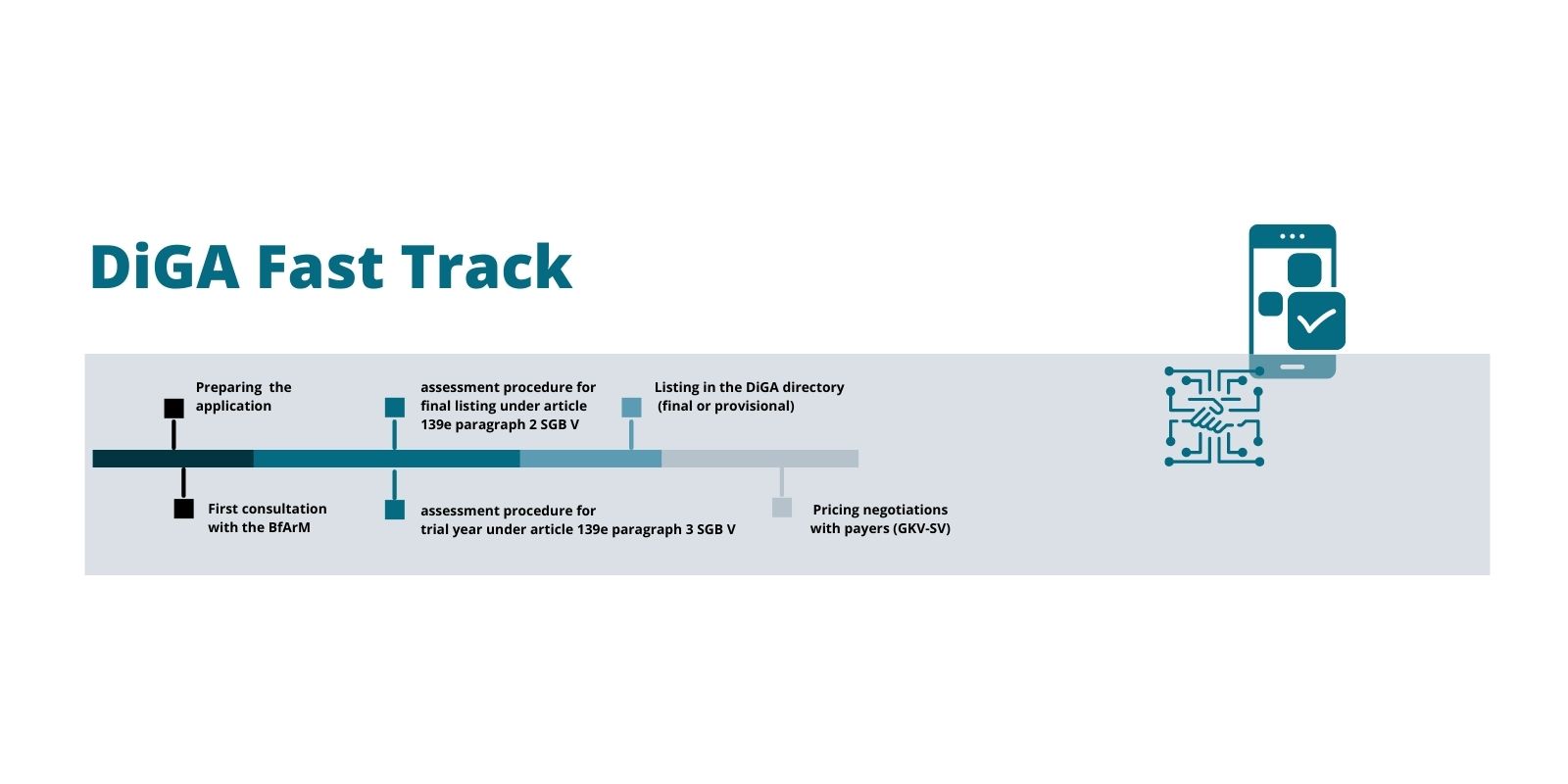
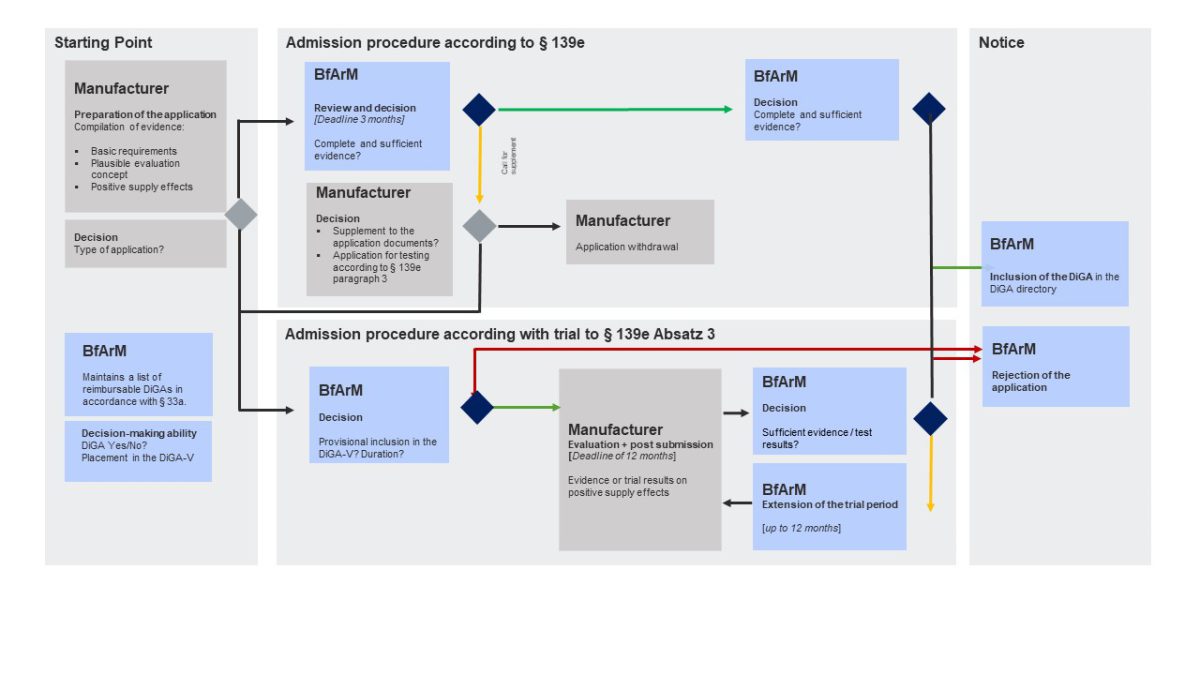
The DiGA Fast Track is the approval procedure of the Federal Institute for Drugs and Medical Devices (BfArM) for the inclusion of digital health applications (DiGA) in the DiGA directory in accordance with Section 139e SGB V. The aim is to carry out an evidence-based and structured review to determine whether a digital application is approved for standard care and therefore eligible for reimbursement.
Below you will find more detailed information on the DiGA Fast Track process, legal and technical requirements and common challenges in the application and approval process by the BfArM.
Digital Health Care Act and DiGA Fast Track
The German Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM) is in charge of DiGA assessment and approval. The BfArM’s assessment procedure opens up a new way for digital health apps to enter the reimbursement market. A DiGA that has successfully completed the assessment procedure is listed in a register of prescription health apps, the DiGA directory.
The DiGA assessment follows a Fast Track to market entry and reimbursement: The BfArM decides on the approval of a digital health app within three months of receiving the manufacturer’s application. Digital health companies can either apply for final or for provisional listing (under article 139e paragraphs 2 and 3 Book V of the Social Code, SGB V).
A provisional listing kicks off a 1-year trial phase after which the manufacturer needs to provide proof of “positive healthcare effects” (positive Versorgungseffekte, pVE) as a basis for the final listing. The DiGA can be prescribed and is reimbursed during the entire trial year. Thus, the DiGA fast track with its possibility of a trial year is a great opportunity for accelerated market access and to offset some of the development costs already during the clinical trial phase. Time to market can be accelerated even more with purposeful planning and preparation.
Criteria Defining a DiGA
Criteria Defining a DiGA
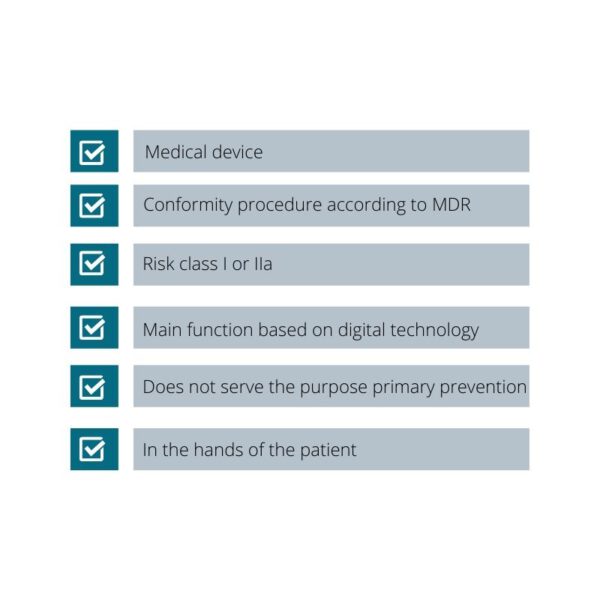
DiGA are a class I or class IIa medical device by European MDR (Medical Device Regulation) classification rules. DiGA support the detection, monitoring, treatment, or alleviation of diseases or the detection, treatment, alleviation, or compensation of injuries or disabilities. A DiGA’s main function is based on its digital technology. A DiGA does not not only serve the purpose of reading out or controlling another (medical) device; a DiGA’s medical intended use is achieved via its digital main function. A DiGA does not serve the purpose of primary prevention. DiGA should mainly be used by the patient, but can also be used by both the patient and healthcare providers together. These criteria are defined in § 33a SGB V.
Regulatory DiGA Requirements
Regulatory DiGA Requirements
To be listed in the DiGA directory under § 139e SGB V, each DiGA needs to conform to the requirements of §§ 3 to 6 of the DiGA ordinance (DiGA-Verordnung, DiGAV).
DiGA need to comply with regulatory requirements pertaining to:
- Safety and suitability for use,
- data protection and information security,
- quality, and in particular interoperability.
The CE marking under the MDR regulation confirms that the digital health app complies with requirements regarding safety and suitability for use. The checklists found in DiGAV annexes 1 and 2 can be used to self-declare compliance with the remaining requirements.
Insights
How to apply the DiGA requirements to a specific product or DiGA concept is not entirely set in stone. Make use of the freedom that both the DiGA ordinance and the DiGA guide leave you. Being well-prepared for the pivotal first consultation with the BfArM maximizes your chances of a successful and speedy approval.
Study Design and Evaluation Strategy
Study Design and Evaluation Strategy
An evaluation concept is the prerequisite of a provisional listing followed by a 1-year trial phase. It has to meet generally accepted scientific standards. The evaluation concept needs to explicate for which type of “positive healthcare effect” (pVE) evidence is to be provided, for which patient population, and why the DiGA is suited achieve the effect. In particular, the evaluation concept should establish the planned study design, the comparator with regard to the day-to-day reality of healthcare, and the chosen trial outcomes. It also needs to specify how evidence is to be produced within the 1-year trial phase.
The evaluation concept must include a study protocol and a statistical analysis plan. It also needs to adequately include and take into account the results of systematic data analysis. Data to be analyzed consist both in a systematic literature research and in data collected with the app itself. The data analysis must demonstrate to the BfArM that the app can achieve the intended positive healthcare effect for the chosen patient group. The analyzed data also provide useful information to define study parameters for the trial period, such as effect size, case number, outcome measurement instruments, and recruitment methods.
The minimum requirement for a DiGA study is a retrospective comparative study. For instance, case-control-studies, retrospective cohort studies, and intraindividual comparisons are all acceptable study types for DiGA. The DiGA manufacturer itself is free to choose a study type of a higher evidence level (see chapter 6 of the DiGA guide). However, the BfArM as well may request a prospective study if the validity of retrospective data is questionable. The DiGA study is to be conducted in Germany. If not, the manufacturer must provide proof that that the healthcare context in which the study was conducted is comparable to the one in Germany. DiGA studies should reflect the reality of German healthcare. The obtained results must be quantitative.
The study is to be registered in a public trial register (e.g., the DRKS register). Complete study results are to be published no later than 12 months after study completion.

Insights
Just like any software, software as a medical device tends to be characterized by agile development, frequent new releases, and tight budgets. Not just for the development of the DiGA software, but also for the evaluation concept, it is key to look ahead and to adapt the original plan if need be. Our recommendations:
- Analyzing and reporting trial data for your application take time. Accommodate for that in your project plan!
- The evaluation concept is an important document to focus on as it is needed for the application. But you cannot focus on the evaluation concept alone. Don’t lose sight of all the other relevant aspects and processes pertaining to market access. Plan ahead!
- When starting to collect data with the app, the app-development should be almost completed. We know from experience that the BfArM requires that the app data are collected with a product that is comparable to the to-be-listed DiGA. Thus, before starting to collect data with your app, make sure that you won’t need to make significant changes later!
Find more insights and learnings in our article „Evaluationskonzept reloaded – Think ahead!“ (in German)
Proof of Positive Healthcare Effects
Proof of Positive Healthcare Effects
With a (final or provisional) listing in the DiGA directory, digital health apps are reimbursable within Germany’s SHI system. One key element of the DiGA Fast-Track assessment procedure focuses on the DiGA’s positive healthcare effect.
The term of positive healthcare effects (pVE) comprises both medical benefits (medizinischer Nutzen, mN) and as patient-relevant improvement of structures and processes (patientenrelevante Struktur- und Verfahrensverbesserungen, pSVV). Before the passing of the Digital Health Care Act, pSVV played no role with regard to the reimbursement of a service or benefit in Germany. With the introduction of pSVV into the German SHI system, lawmakers have endorsed the idea that DiGA can improve healthcare structures and processes and that this type of benefit should justify reimbursement – also in the absence of a medical benefit.
Manufacturers who have already proven their DiGA’s positive healthcare effects in a study can directly apply for a final listing. As an alternative, an application for a provisional listing is possible. The provisionally listed DiGA has 12 months (which can be extended by another year in justified exceptional cases) to prove its positive healthcare effects.
DiGA Pricing
DiGA Pricing
A year after a digital health app has been listed in the DiGA directory, the manufacturer’s price is superseded by a reimbursement agreement between the manufacturer and the National Association of SHI Funds (GKV-Spitzenverband, GKV-SV). If both parties cannot come to an agreement, an arbitration board will set a DiGA price under § 134 paragraph 3 SGB V. The new reimbursement price retroactively takes effect from the thirteenth month after the DiGA listing.
Some principles of DiGA pricing are defined in the master agreement pursuant to § 134 paragraphs 4 and 5 SGB V. For instance, the actual self-pay app price in Germany and from other European countries is factored into the pricing process. In addition, pricing strategies may take into account the number of delivered DiGA activation codes and pay-for-performance principles.
However, the type and amount of proven additional benefit the DiGA can provide for patients remains the most important criterion for DiGA pricing.
Health app reimbursement options beyond DiGA
Health app reimbursement options beyond DiGA
In the German healthcare system, “prescription apps” are not the only reimbursement option for digital health apps. It is often unclear to digital health companies which type of contract is most advantageous from a market access and business perspective.
„Besondere Versorgung“ (§ 140 SGB V) and other product-specific compensation models negotiated with individual payers are commonly used for market access in digital health. But other options to leverage the potential of digital health apps in the outpatient and inpatient sectors as well as in primary prevention should be considered too.
Each market access and reimbursement strategy comes with different stakeholders that need to be approached and convinced, different processes that need to be followed, and different regulatory requirements that need to be met. The time and effort for the application and approval process may also vary considerably from one type of contract to the other.
Therefore, it is important to know your reimbursement alternatives and identify the most appropriate and most advantageous market access option(s) for your product early on, so that you can consider the related processes and requirements right from the start.
We help you find out which compensation and reimbursement models best fit your idea, determine the corresponding requirements, and pave your way through to market entry.
_customer





















_hub
Pointed statements, event reports and concrete how-tos – as magazine articles and videos – can be found here.