DiGA Fast Track: Rapid Access, Smart Strategy – How Digital Innovations Are Conquering the German Healthcare Market

Abstract
Our current beta_shorts #01 „DiGA Fast Track“ with Jonas provides a compact introduction to the DiGA Fast Track: who is involved, how listing in the directory works, and which requirements must be met.
This article goes one step further: it shows why sustainable success requires more than speed – and which strategic decisions companies should make early on.
Key Takeaways
- DiGA Fast Track: Structured access to the statutory health insurance (SHI) market – but demanding requirements.
- Strategic planning beats speed: Consider approval, evidence, and market integration from the very beginning.
- Three success factors: A holistic approach, timing in the product lifecycle, and system understanding.
- Pay attention to market reality: Data from the _fbeta DiGA Analyzer®
- Sustainable success: The Fast Track is the starting point – not the goal.
Planning Beats Speed: Why Strategy is Key in the DiGA Fast Track
The DiGA Fast Track in Germany offers digital health innovations a unique opportunity: the path to reimbursement through statutory health insurance (SHI) is clearly defined, transparent, and achievable within just a few months. For start-ups and pharmaceutical companies, this opens access to a market of over 74 million insured individuals – a model unique on the international stage.
Those who use the Fast Track strategically not only significantly increase their chances of success but also create a sustainable competitive advantage.
Especially in the dynamic field of digital health innovations, it becomes clear: those who think strategically use the Fast Track not only for approval but also as the foundation for sustainable market success.
Successful DiGA manufacturers focus particularly on three aspects:
Holistic perspective on approval and market integration
A DiGA is not just a medical device with a CE mark – it is a care innovation.
Successful use of the Fast Track requires that clinical benefit, user-friendliness, and integration into care pathways are considered together from the very beginning.
Timing in the product lifecycle
It is not the perfect moment of application that matters – but preparation.
The earlier a company integrates evidence-based efficacy and health-economic benefits into its product development, the better it can meet the Fast Track requirements.
Understanding the system context
The Fast Track is more than a regulatory shortcut: it reflects the care logic of the German healthcare system.
Only those who can convincingly demonstrate the benefits for physicians, health insurers, and patients will not only be listed – but also successfully used.
A Reality Check: Insights from the DiGA Analyzer
How dynamic the DiGA market has become is evident when looking at the latest data from the _fbeta DiGA Analyzer. These developments show: The Fast Track opens doors – but real market opportunities emerge where evidence, user-centricity, and economic viability come together.
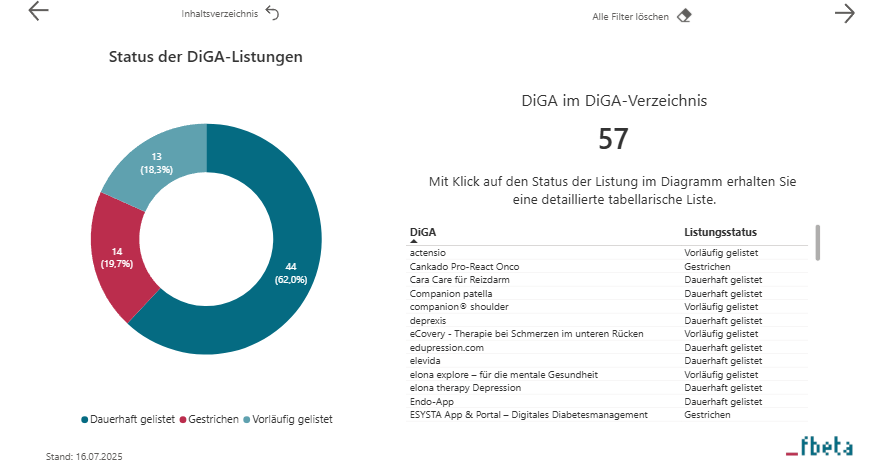
Conclusion: Recognizing Opportunities and Making the Most of the DiGA Fast Track
The DiGA Fast Track is not a shortcut – it is an invitation to bring innovation strategically into healthcare delivery.
With forward-looking planning, solid evidence strategies, and a clear focus on patient care pathways, start-ups and pharmaceutical companies can not only reach the market faster – but also build sustainable success.
_fbeta is your partner on this journey – working alongside you, guided by data, and always focused on the bigger picture.
Further information on the DiGA Fast Track
Contact
Jonas Albert, Partner _fbeta
FAQ – DiGA Fast Track
What is the DiGA Fast Track, and who is it suitable for?
The DiGA Fast Track is an accelerated approval process in Germany for digital health applications that can demonstrate a positive healthcare effect.
It is suitable for start-ups, MedTech companies, and pharmaceutical firms developing innovative digital solutions aimed at rapid integration into standard healthcare.
A DiGA must be classified as a CE-marked medical device in risk class I or IIa and demonstrate positive healthcare effects – either a medical benefit or a patient-relevant improvement in structure and process.
Additionally, compliance with data protection, data security, and interoperability standards is required.
Evidence is a critical success factor for the Fast Track.
Already at the preliminary listing stage, manufacturers must outline how they intend to prove positive healthcare effects.
Companies that plan their studies early and focus on clear endpoints gain a significant strategic advantage.
The DiGA Analyzer provides up-to-date market data on listed and removed DiGAs, price developments, and success factors across different indication areas.
It helps companies identify market trends early, optimize their Fast Track strategies based on data, and better assess potential risks.ng leads start-ups and pharma companies to lasting success.